「No.3」Why does rust cause? 【About Oxidation and Reduction】
As a correct term, “RUST” is used for “Iron” only, while “CORROSION” is used for whole metals including iron. But in this article, it is unified with RUST to make it easier to read for our customers. Also, there is some place which should be explained “xxx MOLECULAR” and “xxx ATOM” in the strict sense.
As the theory of this world, all objects change to a more stable state. For example, if you chill the water at room temperature, it becomes ice at 0°C (from liquid to solid), and steam if heated at 100°C (from liquid to gas). There is no “100°C ice” (solid water) in typical living environment. Because solid water is unstable at 100°C. In high altitude areas such as the top of the mountain, water boils at below 100°C. Because of the effect that the atmospheric pressure has lowered, the water is more stable by changing to steam at 100°C or less (water vaporization).
Then, why the metal rust? The reason is very simple, rusty state is stable, while the state not rust is unstable for metal. Because of the needs of metal in its unstable condition, we have been studying for the rust prevention treatment and rust prevention packaging.
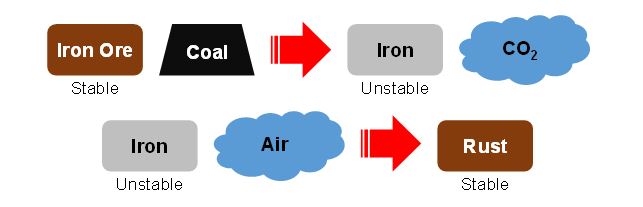
Here, we will explain for the iron as an example. Iron exists in the natural world as an iron ore (stable for iron). There are many iron sulfide states, but the story is complicated, so we omit them in this time. Speaking of the iron ore dare simply, it is a mass of rust. Because rust is composed mainly “iron + oxygen”, “iron” is left by removing “oxygen”. However, as described earlier, because the iron itself is unstable in the natural world, iron ties to return to the state of rust by reacting with the air (stable state for iron).
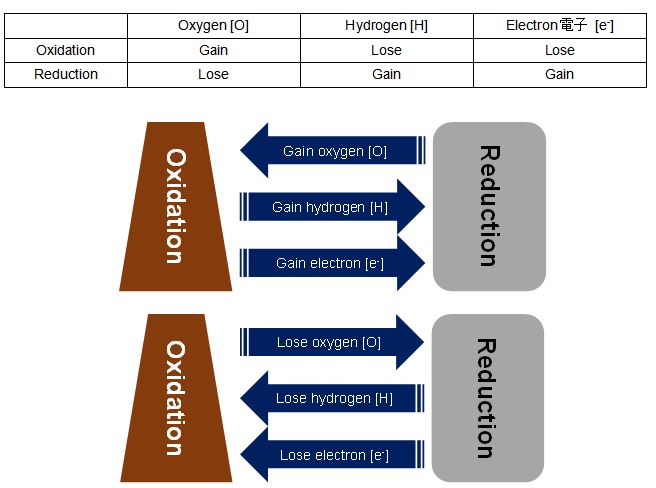
The reaction which becomes rust is “Oxidation-Reduction reaction” which combines “Oxidation reaction” and “Reduction reaction”. This oxidation reaction and reduction reaction are not happened by one of them only. It always happens both. The definition of “oxidation” and “reduction” as follows.
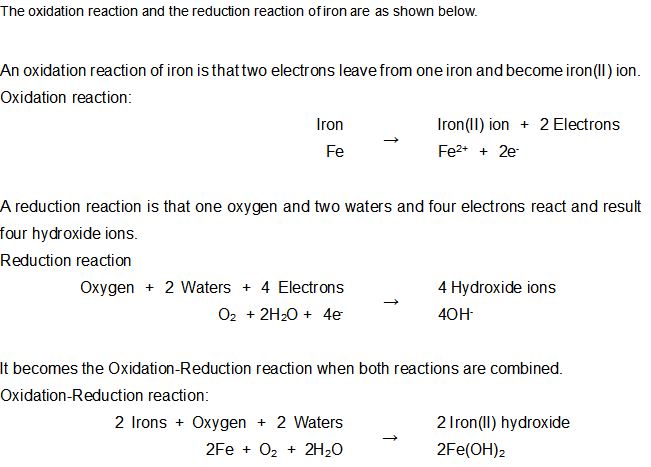
Iron(II) Hydroxide can be made with the resulting of one Iron(II) ion and two Hydroxide ions. It is the familiar “RUST” that this reacts further and becomes Iron(III) Hydroxide.
Simply referred to as “Rust of Iron”, “Iron(II) hydroxide” or “Iron(III) oxide-hydroxide” and ” Iron(II, III) oxide” are different substances that can be made by the environment in which the metal is located, and their properties (colors, etc.) are different.
“Smell of RUST” will be explained in No.5.
